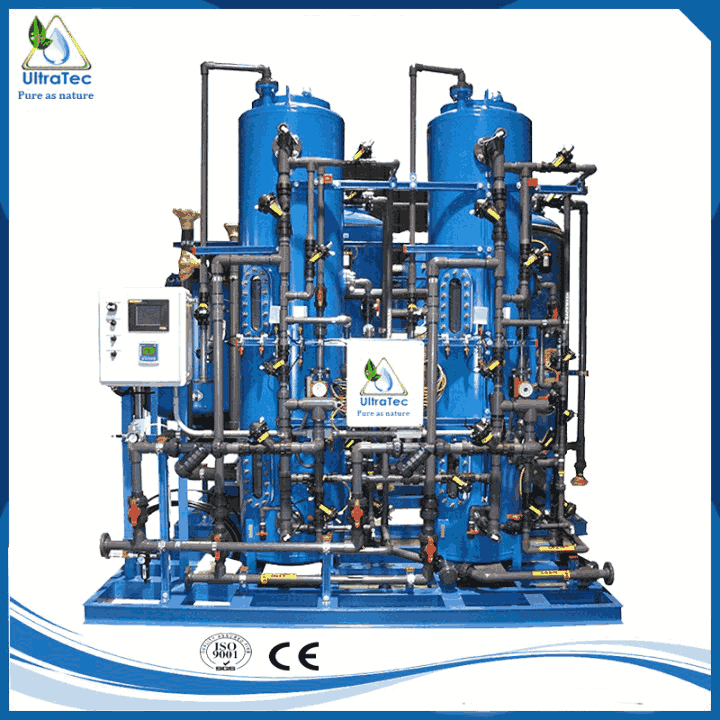
Industrial Separate Bed De Ionizer water, commonly called DI water, is water from which most mineral ions have been meticulously removed. This encompasses cations such as sodium, calcium, iron, copper and anions like chloride and sulfate. DI Water is the preferred water variant across numerous factory and manufacturing environments. Its utilization extends to medical facilities, food processing units, chemical industries, and semiconductor sectors. This spans a spectrum of applications, including industrial cooling processes and the production of cosmetics.
Deionization involves reducing or eliminating positively charged ions, known as cations, and negatively charged ions, referred to as anions, from the water source. This procedure can generate water of exceptional purity, displaying conductivity measurements ranging from 50 to 1 micro Siemens per centimeter or achieving an even superior outcome by utilizing commonly accessible water sources. The cation resin transforms into the hydrogen form, effectively exchanging positive ions for hydrogen ions. On the other hand, the Anion resin changes into the hydroxyl form, effectively swapping out negative ions for hydroxyl ions.
Specially designed resins are tailored for deionizer purposes, typically regenerated through the utilization of 6.4 lbs of 100% hydrochloric acid (HCI) per cubic foot for cation resin and 8.0 lbs. of 100% sodium hydroxide (NaOH) per cubic foot for anion resin. Lakeside resins offer exceptional chemical and physical stability, reducing pressure drop and boasting remarkable resistance to bead breakage. Each customer’s distinctive water quality needs dictate the selection between solid and weak base resins. Selecting Type 1 or 2 Anion resins hinges on the specific application’s silica residual demands. In standard setups, pressure-compensating regulators control the intake of chemicals, RO Plant water treatment companies in UAE.
The Mix Bed De Ionizer
DE-IONIZER eliminates or minimizes positive ions, cations, and negative ions, known as anions, from the water source. It generates high-purity water ranging from 1.0 to 18.3 megaohms/cm and achieves a silica level of either .10ppm to 0.01ppm or even lower. The cation resin transforms into its hydrogen form, facilitating the exchange of positive ions for hydrogen ions. Meanwhile, the Anion resin converts into the hydroxyl form, engaging in the business of negative ions for hydroxyl ions.
The resins undergo a regeneration process specifically tailored for deionization applications, utilizing approximately 6.4 pounds of 100% hydrochloric acid (HCI) per cubic foot for cation resin and about 8.0 pounds of 100% sodium hydroxide (NaOH) per cubic foot for anion resin. The resins provided by Lakeside exhibit exceptional chemical and physical stability, ensuring minimal pressure drop and displaying remarkable resistance to bead breakage. Type 1 Anion resin is the norm for minor silica leaks. Whereas 10% cross-linked cation resin finds its application for utmost durability and performance by UltraTec®.
The standard approach for Cation regeneration involves using 32% hydrochloric acid (HCl). In formal systems, operators utilize pressure-compensating educators to extract the required chemicals. To prevent corrosion, it is recommended to maintain the combined chloride and sulfate content in the dilution water below 25 ppm. The presence of complex minerals and salts in the dilution water can amplify. The occurrence of metal corrosion and contribute to the development of scale and deposits.
Specification Industrial Separate De Ionizer:
DI stands for Demineralization through Ionization, wherein a singular DI filter effectively eliminates the scant mineral ions that manage to pass through the RO membrane. This meticulous process ensures the highest degree of water purity achievable. Notably, the DI filter surpasses the RO membrane’s capacity by extracting nitrates that remain unaffected during the initial filtration. The core of the DI filter consists of a cartridge densely packed with ion exchange resin. Taking on the form of diminutive beads. As water courses its way alongside these beads, the minerals, and assorted ions undergo separation from the water. Adhering to the surface of the beads.






Reviews
There are no reviews yet.